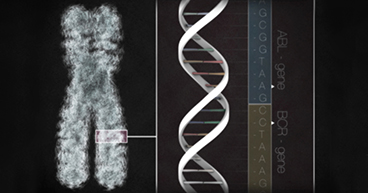
At the root of most cancer diagnoses is a biopsy, the surgical procedure used to remove a tumor so it can be examined for the presence of cancer cells. Doctors often rely on biopsies to help make an assessment of a cancer's malignancy, stage, origin and DNA mutations that may be targeted with treatments. Some biopsies are minimally invasive; others may be more challenging, requiring stitches or anesthesia. Now, a simple blood test called a liquid biopsy offers another tool that may give doctors a more complete profile of solid tumors. The test is not a substitute for surgical biopsies, but instead may offer additional information to help identify specific treatment options. While this evolving technology is being used only on specific lung cancers right now, it is being explored as a profiling and monitoring tool for other types of solid tumors. "The term liquid biopsy is a new concept in which we analyze bodily fluids and look for DNA alterations that might indicate cancer, or for specific genetic features that may be driving a cancer's growth," says Arturo Loaiza-Bonilla, MD, MSEd, FACP, Chief of Medical Oncology and Medical Director of Research at our hospital in Philadelphia.
Testing for cancer cells
Tumors are constantly shedding cells, which carry their genomic blueprint. These cells often wind up in the bloodstream. Liquid biopsies are designed to isolate these cells and look for certain genetic features that may indicate cancer and what mutations may be driving the tumor's growth. "In the majority of cancer-related tests, these liquid biopsies are looking for circulating tumor DNA—fragments found in the bloodstream of cancer patients," says Dr. Loaiza-Bonilla. "The test may find that some fragments have mutations that are specific to cancer, and may help identify weaknesses we may target to improve treatment options."
The U.S. Food and Drug Administration last year approved the first blood test to detect a gene mutation found in some patients with non-small cell lung cancer. The test, called the cobas® EGFR Mutation Test v2, is designed to identify mutations in the epidermal growth factor receptor (EGFR) gene without requiring a tissue sample. "A liquid biopsy may detect this mutation rather quickly and efficiently so that a patient may benefit from specific treatment that targets the mutation," says Dr. Alan Tan, Medical Director of Hematology and Immunotherapy and a Medical Oncologist and Hematologist at our hospital near Phoenix.
In most cases, liquid biopsies refer to blood tests, but they may also involve other types of specimens, including urine, stool or spinal fluid. Cologuard®, for instance, is a type of stool test that may offer an alternative to a colonoscopy for some patients, especially those with lower risks for colorectal cancer. Cologuard differs from other liquid biopsies, Dr. Tan says. "Cologuard also looks to find elevated levels of altered DNA, which could be associated with cancer or pre-cancer," he says. "With Cologuard, we are not looking at specific driver mutations but genetic features that that may provide you with a positive or negative result for cancer."
Doctors in Canada, working with researchers at the Eastern Virginia Medical School, say they may be close to developing a urine test that may detect proteins present only in prostate cancer patients. The test may also reveal whether cancer is aggressive, researchers say. "We believe we have found a better way that allows us to predict which patients have a slow-growing versus aggressive prostate cancer using non-invasive biomarkers," principal investigator Thomas Kislinger, Senior Scientist at the Princess Margaret Cancer Centre and Associate Professor at the University of Toronto, told Science Daily. "This could eventually help us personalize cancer treatment for these patients."
Liquid biopsy limitations
But liquid biopsies also have their limitations. In some cases, further procedures may still be required after a liquid biopsy to obtain a more detailed diagnosis. Liquid biopsies are also limited in how many cancers they may be used to analyze. Current tests don't detect most DNA mutations, and some tumors don't shed enough cells into the bloodstream to be detected.
If it is eventually developed as an alternative to surgical biopsies, the liquid biopsy has some clear advantages, Drs. Tan and Loaiza-Bonilla say. "The most obvious benefit would be the convenience and safety of not requiring an invasive procedure," Dr. Tan says. And in cases of metastatic cancer, a liquid biopsy may also offer doctors a broader picture of a patient's overall cancer challenge. "Because tumor cells mutate and change their DNA as they travel through the body, taking a blood sample may show us DNA fragments coming from all over, unlike a biopsy of a single site," says Dr. Loaiza-Bonilla.
Dozens of clinical trials are being conducted to determine whether liquid biopsies may be a potential diagnostic tool for a variety of cancers, including pancreatic, kidney and ovarian cancers. "The verdict is still out on whether liquid biopsies will replace traditional biopsies," Dr. Tan says. "As physicians, we do not want to subject our patients to unnecessary discomfort and anxiety with invasive procedures. So, if there is a technology to replace that without sacrificing quality, then it’s a no-brainer."